Tooth loss and bone degeneration have long remained some of the toughest challenges in medicine, but new research suggests that science may be closer than ever to reversing both. A study published in Nature Communications in 2025 has identified two key types of stem cells—and the genetic mechanisms that control them—that could eventually allow the human body to regenerate lost teeth and rebuild bone tissue.
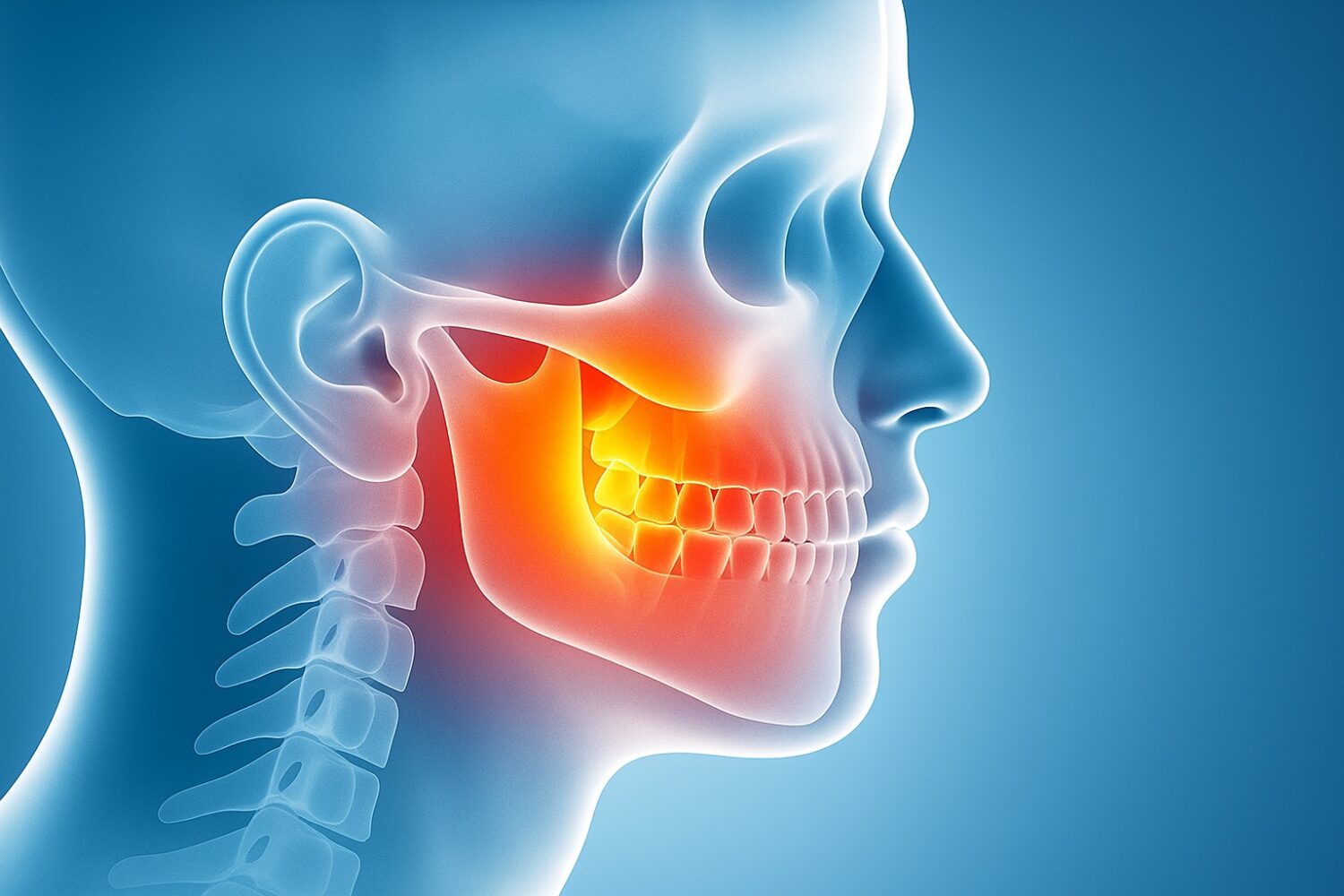
The findings center on the alveolar bone, the structure that anchors teeth and absorbs the pressure from chewing. This bone is particularly vulnerable to wear and tear, and its deterioration is a major factor in periodontal disease, which affects billions worldwide. In the U.S. alone, roughly 10 million adults over 50 have osteoporosis, and the CDC estimates that more than one in ten seniors have lost all of their teeth. The potential to regenerate this bone could dramatically improve both oral and skeletal health.
Researchers from the Nature Communications study, titled “A Hedgehog–Foxf axis coordinates dental follicle-derived alveolar bone formation,” used genetically engineered mice to trace how certain dental stem cells develop. By tracking PTHrP-expressing cells in the dental follicle, they found that these cells mature into osteoblasts and osteocytes—the bone-building and bone-maintaining cells responsible for forming the alveolar region around developing teeth.
Central to this process is a genetic signaling mechanism known as the Hedgehog pathway, which governs how stem cells grow and differentiate. The study revealed that while Hedgehog-related genes are active early in tooth and bone formation, they must deactivate at the right stage for normal bone development to occur. When this pathway remained switched on for too long, bone growth became abnormal—a process that could help explain degenerative jaw and gum diseases.
The researchers also compared healthy and diseased bone tissue and found that in cases of periodontitis, the Hedgehog pathway reactivated during bone loss, disrupting normal cell behavior. Crucially, treatment with LDE225, an FDA-approved drug that blocks Hedgehog signaling, restored normal bone formation in animal models without damaging healthy tissue. This suggests that the same mechanism could one day be harnessed to regenerate bone in humans.
Further analysis identified the role of another gene network, known as the Fox family, which regulates facial and skeletal development. The team discovered that one gene in particular, Foxf1, becomes overactive when Hedgehog signaling is misregulated. Reducing Foxf1 activity restored healthy bone growth, indicating that Foxf1 serves as a key intermediary between genetic signals and the physical process of bone regeneration.
Together, these findings mark a major step forward in understanding how dental stem cells can be controlled to rebuild bone and potentially regenerate teeth. While clinical applications remain years away, this research lays the groundwork for targeted therapies that could combat bone loss, gum disease, and even conditions like osteoporosis. When combined with other genetic discoveries—such as work on the GPR133 gene, which may also help reverse bone density loss—the emerging picture points toward a future where regenerating bone and dental tissue could move from laboratory theory to medical reality.